GPs often receive echocardiogram reports and are asked to interpret them. Here is a grossly simplified version of how. I have concentrated on explaining the “jargons” on the echocardiogram report in plain physiology, hence the length of the article!
Roughly speaking, echocardiogram uses ultrasound waves to
visualise cardiac structures – in 2D or 3D
measure flow velocities via Doppler
While there may be different echocardiogram report formatting, the interrogated structures are either cardiac chambers or cardiac valves.
Systematically, therefore, the report will comment on the left ventricle, right ventricle, left atrium, right atrium; mitral valve, aortic valve, tricuspid valve, pulmonary valve; as well as the visualised aorta, superior vena cava, inferior vena cava, and pericardium.
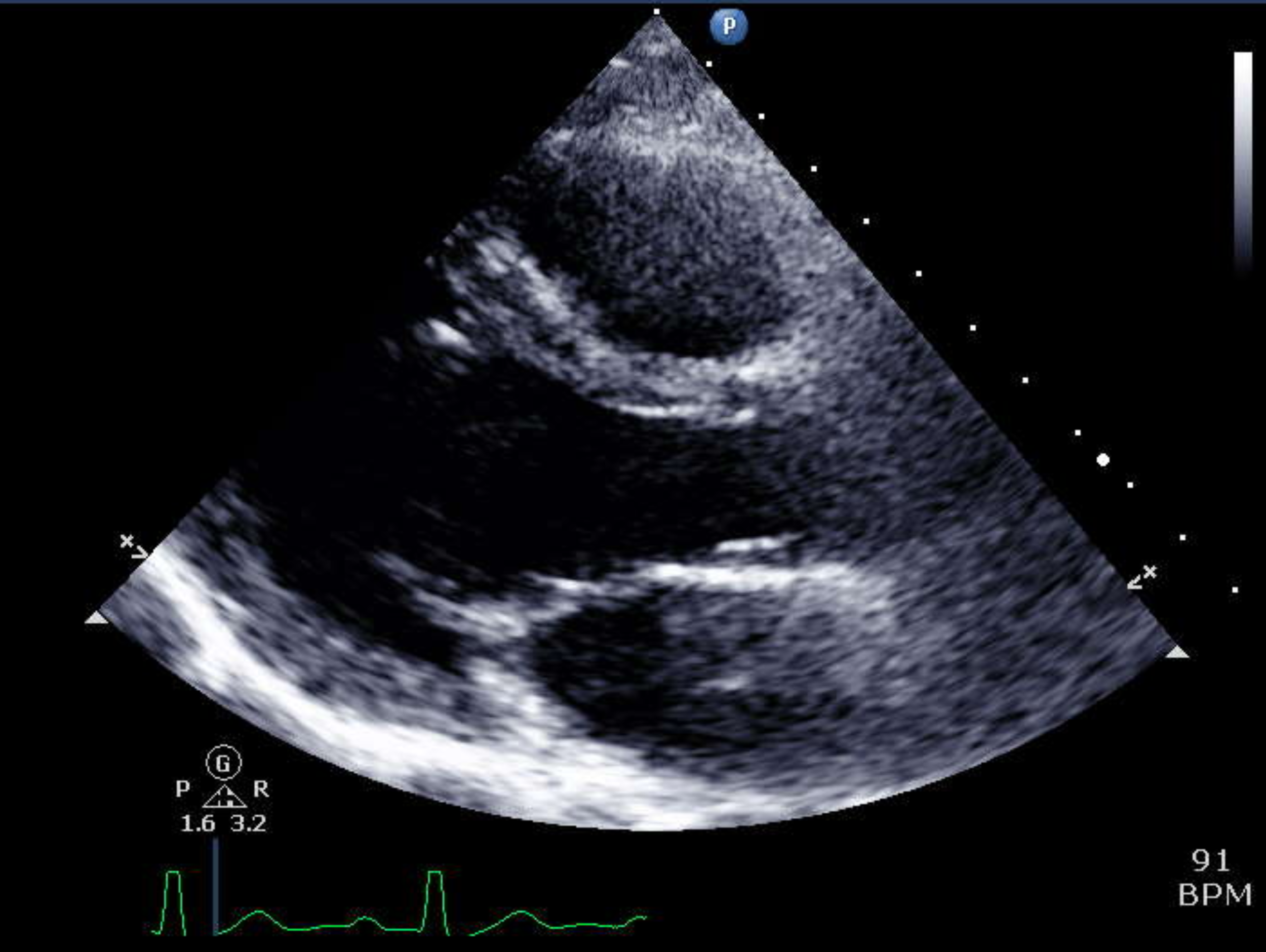
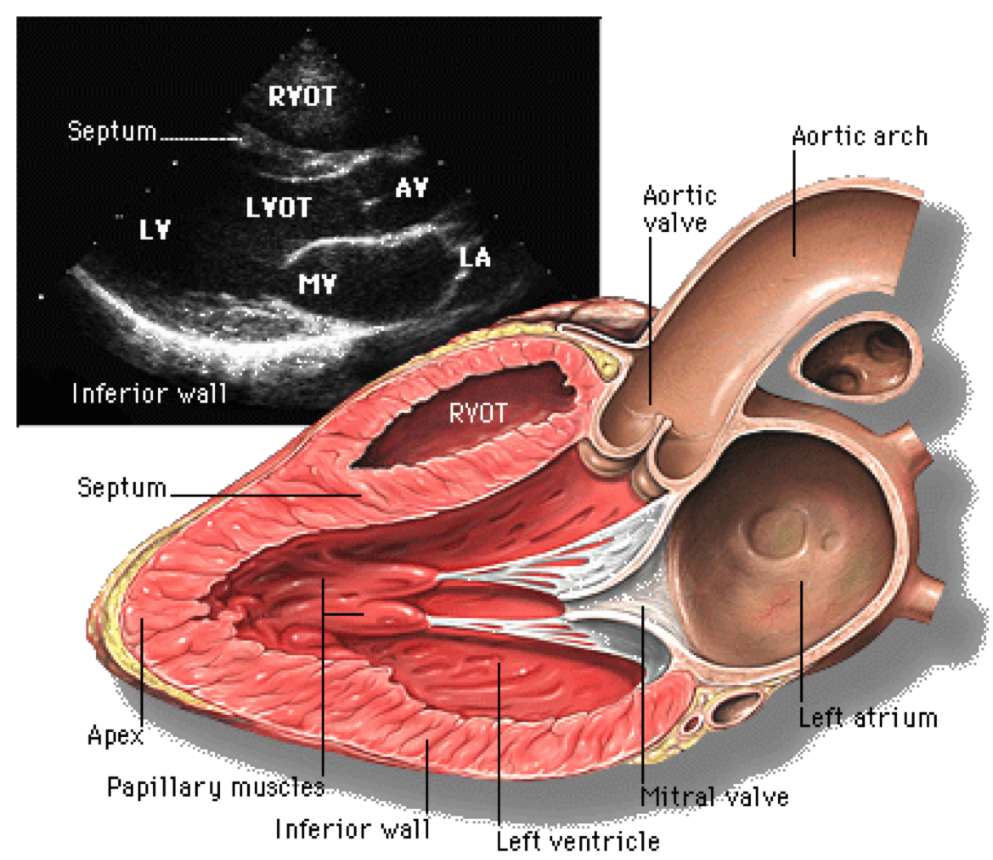
Parasternal long axis view of the heart, as an illustration of how echocardiogram assesses various cardiac chambers, and heart valves. Ref: Yale Atlas of Echocardiography.
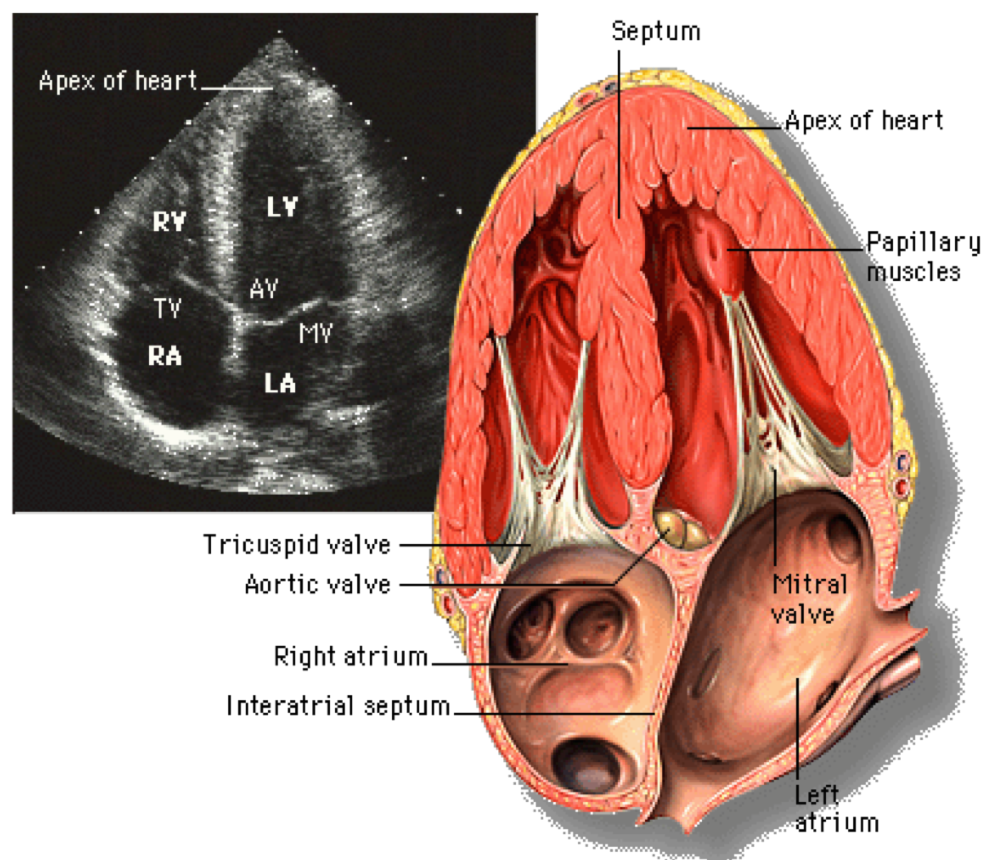
Apical 4-chamber view, giving us information of the left ventricle, left atrium, mitral valve; right ventricle, right atrium, tricuspid valve, and often aortic valve.
Almost all abnormalities are graded mild, moderate, or severe. Complexity of the echocardiogram report lies in the inclusion of clinically useful parameters that justify the conclusion about the various structures. However, if we concentrate on the main themes, we can definitely decipher the main messages.
Cardiac chambers
Assessment of cardiac chambers basically entails their size and function. Practically, assessment of function is limited to the ventricles.
Left ventricular size
Left ventricular size could be assessed by linear measurements (LVEDD/LVESD), volume measurements (LVEDV, LVESV) using 2D images, or 3D volume measurements. In some ways, understanding the exact methods for deriving these measurements may not be as important. However, in general, volume measurements are more accurate, reliable and reproducible than linear measurements.
Clinically, the use of linear measurements remains important for valvular heart disease follow-up, as surgical thresholds remain firmly defined by linear measurements, namely end diastolic diameter (LVEDD) and end systolic diameter (LVESD).
Left ventricular systolic function
Calculating LV ejection fraction requires tracing the endocardial borders at diastole and systole. Ref: Ciampi and Villari, Cardiovascular Ultrasound 2007; 5:34.
Left ventricular systolic function is quantified via the measurements of left ventricular volume at end-diastole and end-systole, with the difference being is the stroke volume (SV). Therefore, the fraction of the amount of blood ejected during each cardiac cycle to the overall end diastolic volume gives us the left ventricular ejection fraction, LVEF. Left ventricular ejection fraction above 55% is generally considered normal whereas ejection fraction below 35% is generally considered severely impaired.
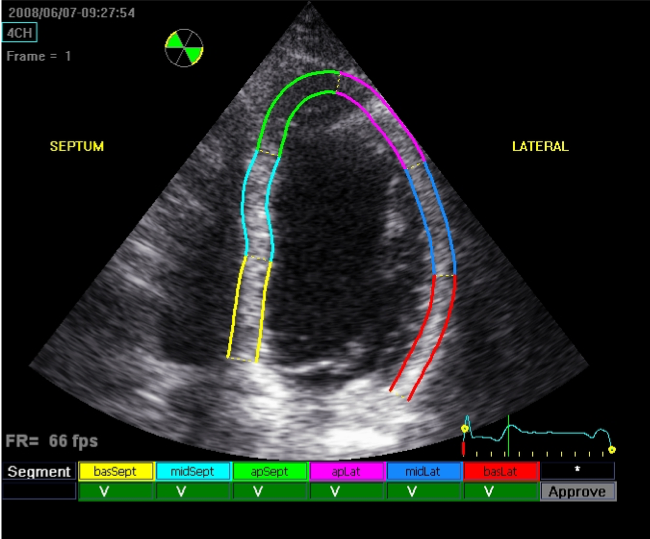
Newer methods of left ventricular systolic function assessment include global longitudinal strain (GLS). This is a more sensitive method of detecting mild changes in LV contractilty, and therefore is the gold standard for assessing subclinical changes in LV function, important for chemotherapy surveillance. Myocardial strain assessment involves estimating regional myocardial contractility directly by tracking speckles produced on ultrasound.
Speckle tracking is used to measure myocardial strain. This is now routinely performed.
Left ventricular diastolic function
Left ventricular diastolic function assessment is incredibly complex. Details of the parameters are therefore not important in this.
Clinically, the presence of significant diastolic dysfunction may tell us the possibility of diastolic heart failure as the cause of the patient’s shortness of breath, although the diagnosis remains one of exclusion and therefore relies on careful clinical assessment.
“Restrictive diastolic filling” or “severe diastolic dysfunction” in the context of both ischaemic heart disease and congestive heart failure is very poor prognostic sign.
“Elevated filling pressures” may call for escalating diuresis, but one needs to be cautious about potential inadequacy of the parameters and therefore one should not rely on this alone to make clinical decisions.
Right ventricular size
Right ventricular size assessment is difficult and therefore largely subjective. Right ventricular end diastolic diameter (RVEDD) is sometimes quoted.
Right ventricular systolic function
Right ventricular systolic function assessment is also subjective. Normal ranges for parameters are therefore not important for this discussion.
Frequently used parameters include
TAPSE (Tricuspid annular plane systolic exclusion) - describe the extent of movement of the tricuspid annulus towards the ultrasound probe
S’ - describes velocity of tricuspid annulus movement towards the ultrasound probe
RV free wall strain - uses strain analysis to look at right ventricular free wall contractility directly
Pulmonary pressures
Assessing pulmonary pressures mainly relies on the presence of the tricuspid regurgitation jet, allowing us to calculate the pressure difference between the right ventricle (hence pulmonary artery) and the right atrium (which we estimates clinically via the jugular venous pressure, or with echocardiographic clues).
There are indirect signs of pulmonary hypertension, which are sometimes referred to in the report.
Left atrial size
Left atrium can be assessed by linear measurement, area measurement and volume measurement.
Left atrial size is prognostic in a number of clinical conditions.
Left atrial volume has largely replaced the other measurements as the gold standard, as it is more prognostic than the other measures.
Aorta
Echocardiogram visualises aortic root, which includes aortic sinuses and sinotubular junction, the very proximal ascending thoracic aorta, sometimes aortic arch, and often the proximal descending thoracic aorta.
Comments about these structures mainly surround size, and aortic wall calcifications.
Any dilation beyond 50 mm would be important, as 55 mm is commonly the surgical threshold, unless an aortopathy calls for an even lower threshold.
Cardiac valves
Assessment of cardiac valves mainly entails the aetiology and severity of heart valve lesions. Again, parameters are quoted to support the conclusion. Hence, parameters are important but difficult to understand, while the conclusion should be straightforward.
Mitral valve aetiology
Mitral valve disease could be caused by primary valvular pathology or secondary to what happens in the left ventricle.
Primary mitral valve pathologies are varied, including mitral valve prolapse with or without flail, rheumatic heart disease, endocarditis. Treatment, if severe, is invariably surgical.
Mitral valve prolapse describes the bellowing of the valve apparatus beyond the mitral annulus plane, defined by the imaginary line between the lateral mitral annulus and aortomitral continuty (where the aortic valve apparatus joins the mitral valve apparatus). Therefore, varying degree of mitral valve prolapse could be associated with varying degree of mitral regurgitation.
The presence of flail simply describes part of the mitral valve apparatus, be it the leaflet itself, or the subvalvular chords, pointing directly towards the left atrium. Anatomically, it means that there has been disruption of the valvular apparatus holding the leaflets onto the left ventricle.
Secondary mitral valve pathologies are mostly due to changes in the left ventricle that usually holds the mitral apparatus intact. The two main categories include:
Functional mitral regurgitation, resulting from the general dilation of the mitral annulus, thereby stretching the co-optation plane apart. Mitral regurgitation is usually central.
Ischaemic mitral regurgitation, resulting from the localised dysfunction of the lateral wall of the ventricle, thereby affecting the posterior leaflet mechanism. Mitral regurgitation is usually eccentric, posteriorly directed.
While primary mitral valve disease requires surgical treatment, treatment of secondary mitral regurgitation is controversial, mainly reflecting the fact that treatment should be concentrated on the left ventricle itself.
Mitral regurgitation severity
There are numerous parameters and measurements used to estimate mitral regurgitation severity. They centre on the assessment of
mitral regurgitation jet, its appearance, its depth of penetration, its width
effect of the mitral regurgitation on pulmonary flow
While these may be described in the conclusion of the echocardiogram report, they are not in generally useful for GPs.
The most important parameter for mitral regurgitation is in fact left ventricular size, which is the major determinant of when surgery is needed for severe mitral regurgitation.
Aortic valve assessment
Aortic valve can either be stenotic or regurgitant. Again, aetiology and severity are the two key aspects.
Aortic stenosis is mainly degenerative, but could also be caused by bicuspid aortic valve. Aortic stenosis severity relies on the assessment of its flow characteristics. In flow dynamics and the Bernoulli’s equation, the more severe the aortic valve stenosis is, the higher the associated velocities and gradients across the valve. Severe aortic stenosis usually has a peak velocity of over 4.0 m/s and a mean gradient of over 40 mmHg. aortic valve area could be derived by the continuity equation in an area of less than 1.0 cm² would be considered severe.
The Bernoulli’s principle (for those who love Maths), explaining why flow velocities increase across a narrowed valve.
Aortic regurgitation assessment is similar to mitral regurgitation. Aetiology is varied, but treatment is all surgical. Severity assessment relies on the jet characteristics and the effect of aortic regurgitation on ascending thoracic aortic flow. Again, parameters are often quite complicated and beyond the scope of this document. Left ventricular size again an important determinant of when surgery is needed. Concomitant aortic sinuses dilation and ascending thoracic aortic dilation are important to consider.
Tricuspid regurgitation
Tricuspid regurgitation assessment is similar to mitral regurgitation, again concentrating on severity, and aetiology. One of the major Doppler interrogation of tricuspid regurgitation involves using it to estimate pulmonary artery pressure.